Children are known for insatiable curiosity that manifests itself with endless questions of, “What is that?”, “How does that work”, and quite often the simple ponderance, “Why?” Although this behavior is most obvious in our developmental years, I can assure you that adults continue to ask these fundamental questions as I attempt to field responses regarding our scientific mission here in Antarctica.
Children are known for insatiable curiosity that manifests itself with endless questions of, “What is that?”, “How does that work”, and quite often the simple ponderance, “Why?” Although this behavior is most obvious in our developmental years, I can assure you that adults continue to ask these fundamental questions as I attempt to field responses regarding our scientific mission here in Antarctica.
Apart from the numerous questions I receive regarding Antarctica itself, the most common question concerns the purpose of our visit to this southern land. In a word, the answer is: ozone. While the stable, diatomic (O2) form of oxygen is undoubtedly everyone’s favorite atmospheric constituent due to our respiratory dependence on the molecule, its lesser known cousin, ozone, also plays a critical role in our atmosphere. Generally speaking, ozone is created when a single oxygen atom combines with our usual, diatomic (O2) oxygen to produce triatomic (O3) oxygen, better known as ozone.
![]() When you hear the words Antarctica and ozone in the same sentence, you almost certainly think about the infamous “hole in the ozone layer” above the continent. The ozone of this context exists tens of miles above the Earth in the stratosphere and is naturally created when ultraviolet light from the sun strikes O2 freeing up two oxygen atoms that then combine with O2 molecules to create O3. The specific size of the ozone molecule makes it a perfect optical filter for harmful wavelengths of ultraviolet (UV) light from the sun, without which we are subject to intense solar radiation linked to sun burns and even been skin cancer. This topic became wildly popular in the 1980’s when the “ozone hole” over Antarctica was discovered and ultimately linked back to chloroflourocarbons (CFCs) that act to catalyze the destruction of ozone back into diatomic oxygen (O2). Subsequent to this finding, CFCs were banned from aerosol hairsprays and spray paint, but the annual ozone hole remains until this day as the CFCs persist in the stratosphere. While the study of the Antarctic ozone hole is a fascinating subject worthy of in-depth study and discussion in its own right, the purpose of our Antarctic expedition has little to do with stratospheric ozone or the ozone hole.
When you hear the words Antarctica and ozone in the same sentence, you almost certainly think about the infamous “hole in the ozone layer” above the continent. The ozone of this context exists tens of miles above the Earth in the stratosphere and is naturally created when ultraviolet light from the sun strikes O2 freeing up two oxygen atoms that then combine with O2 molecules to create O3. The specific size of the ozone molecule makes it a perfect optical filter for harmful wavelengths of ultraviolet (UV) light from the sun, without which we are subject to intense solar radiation linked to sun burns and even been skin cancer. This topic became wildly popular in the 1980’s when the “ozone hole” over Antarctica was discovered and ultimately linked back to chloroflourocarbons (CFCs) that act to catalyze the destruction of ozone back into diatomic oxygen (O2). Subsequent to this finding, CFCs were banned from aerosol hairsprays and spray paint, but the annual ozone hole remains until this day as the CFCs persist in the stratosphere. While the study of the Antarctic ozone hole is a fascinating subject worthy of in-depth study and discussion in its own right, the purpose of our Antarctic expedition has little to do with stratospheric ozone or the ozone hole.
 Quite apart from the study of lofty stratospheric ozone, the other atmospheric location of interest for ozone study is in the layer of air in closest contact with the Earth’s surface known as the troposphere. Quite appropriately, it is termed ground level ozone or surface level ozone, and this is the subject of our scientific study here in Antarctica. Before considering ground level ozone in polar regions, it’s worth considering what we already know about ground ozone from our own direct experiences. From a sensory standpoint, we are all intimately familiar with the unique smell of newly created ozone after lightning accompanies a rain storm and which we associate with the fresh smell of rain. In a less positive sense, we often heed summertime warnings from news stations who report “dangerously high levels of ozone” and recommend that we fill our gas tanks in the evenings to avoid exasperating the problem.
Quite apart from the study of lofty stratospheric ozone, the other atmospheric location of interest for ozone study is in the layer of air in closest contact with the Earth’s surface known as the troposphere. Quite appropriately, it is termed ground level ozone or surface level ozone, and this is the subject of our scientific study here in Antarctica. Before considering ground level ozone in polar regions, it’s worth considering what we already know about ground ozone from our own direct experiences. From a sensory standpoint, we are all intimately familiar with the unique smell of newly created ozone after lightning accompanies a rain storm and which we associate with the fresh smell of rain. In a less positive sense, we often heed summertime warnings from news stations who report “dangerously high levels of ozone” and recommend that we fill our gas tanks in the evenings to avoid exasperating the problem.
Once again, although the molecule in these circumstances is identical to the one we are here to study, our familiar context is also generally unrelated to the surface level ozone phenomenon in Antarctica. Our familiar experiences are based on its presence in the mid-latitudes that is defined by a rich, complex environment that is inextricably tied to anthropogenic (man-made) influences. As you can imagine, with such a wide variety ozone creation sources and depletion mechanisms, it is incredibly challenging to ascertain the exact atmospheric chemistry that is in action. But imagine the antithesis, a location almost entirely independent of anthropogenic effects; a place that that feels like the clock was turned back on geologic time scales to an almost unrecognizable setting ideally suited to study natural processes without the complications of the post-industrial era; this is Antarctica.
If surface level ozone concentrations were a naturally constant value in the Antarctic, research would have concluded years ago, but as with most interesting stories, there is much more to be told. In fact, previous studies in the Arctic (northern polar regions) detected annual springtime depletion of surface level ozone which was later confirmed by short term studies in the McMurdo region of Antarctica. These mysterious depletion events were confounded by the addition of significant Arctic pollution but simultaneously raised eyebrows as direct corollaries to the stratospheric, springtime depletion events of the ozone hole. Furthermore, Arctic surface level ozone has been considered a potentially significant greenhouse gas in the region of the Earth that is most critically linked to global warming. Clearly, a more thorough understanding of this fundamental atmospheric chemistry was needed.
One day, amidst the sweltering summer heat of 2011, I began a conversation with my friend and LASP colleague, Lars Kalnajs, that I expected would follow our usual twists and turns from outdoor exploration to integration of Arduino microcontrollers into our vehicles. But to my surprise, he informed me that he and Linnea Avallone had received a grant from the National Science Foundation (NSF) to perform a first-of-its-kind, long-duration, distributed study of surface level ozone in Antarctica, and then he shocked me by extending an invitation to assist with the field installation of these instruments during January/February of 2012. After confirming my response to join this endeavor, I began to learn about the various scientific and engineering minutia required to perform these measurements.
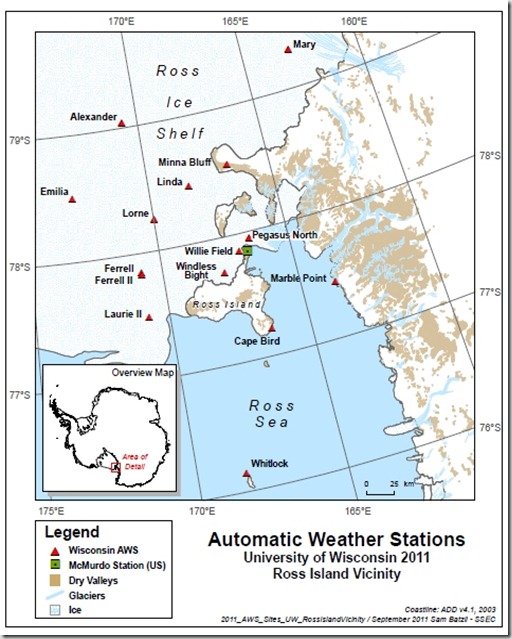
In particular, previous studies seemed to indicate that the annual, springtime depletion of polar surface level ozone was related to extreme cold conditions near sea ice that allows nonreactive halogens (bromine) to become reactive catalysts for the destruction of ozone in an analogous manner to the extreme cold of polar stratospheric clouds that participate in ozone hole destruction. However, the specifics of whether this destruction was occurring locally, in direct contact with the sea ice or in a distributed manner was simply unknown. This fundamental, spatial question provided the impetus to simultaneously measure ozone across a network of sites that could be clearly understood in relation to each other. Fortunately, the University of Wisconsin operates a large network of automatic weather stations (AWS) throughout Antarctica that provide continuous data to atmospheric models of the area. In order to leverage these mature sources of data while providing a spatial distribution away from the sea ice, the instrument network was architected to place four ozone instruments adjacent to existing weather stations.
The instrument location closest to the sea ice is at the northern tip of Ross Island called Cape Bird where it is expected that representative depletion events commence. Then moving away from this source is a location on the main continent called Marble Point for the second station and moving farther along the continent is Minna Bluff for the third station. Finally, far removed from Cape Bird but isolated on the Ross Ice Shelf is Lorne, the site of the fourth station. Each of these sites already consists of an AWS large,steel tower with an anemometer, temperature sensors, assorted other meteorological devices, radio telemetry, a small solar panel, and a bank of rechargeable batteries.
Since the ozone depletion events occur annually and are measured relative to annual baseline values, the ozone instrument must perform measurements throughout the year and on a frequent basis. And unlike the relatively quick and simple measurement methods of the AWS, ozone measurement is far more complex with vacuum pumps, lasers, highly sensitive detectors, and specialized computers that orchestrate the activity. All of this technical complexity is usually encompassed in large, laboratory grade instruments that casually consume gobs of 120VAC wall power. However, on a continent whose environmental superlatives are comparable to that of deep space, instrument design becomes an even more formidable challenge. The ozone instruments for this campaign were custom designed, manufactured, assembled, and calibrated by Lars himself which is a technical feat considering that such endeavors are typically the products of entire teams of engineers and scientists. And while the instruments are optimized for minimal size and power consumption, they still require respectable amounts of power to run throughout the cold, dark months of the Antarctic winter. Fortunately, the AWS folks and another seasoned polar expert, UNAVCO, have previously addressed these challenges and were able to provide time-tested power system infrastructures for the ozone instruments. In contrast to the unassuming, boxy appearance of the ozone instruments, the power systems are in-your-face with large, aluminum, triangular frames that house two 80 watt solar panels, two compact wind turbines, and two waterproof Hardigg cases. And within these Hardigg cases are 12 sealed lead acid batteries, power distribution and charging electronics, and finally, the piece de resistance, the ozone instrument.
It is amazing that something so small and simple as an O3 molecule could inspire such dedicated efforts and studies, but the need to discover and learn is among our deepest human desires and like the Antarctic explorers who preceded us, huge efforts are clearly required to reach meaningful accomplishments. With each day we spend on the continent, we push harder and closer to our expedition’s goal to deploy the ozone monitoring network, so stay tuned to hear about the progress we have achieved during our time in Antarctica.